Free CBSE Science Notes for Class 10, Chemistry Chapter Acids, Bases and Salts, CBSE Exam 2024 Best Study Materials for Students.
Acids Bases And Salts
There are a large number of chemical compounds, which are classified into three groups based on chemical properties. These groups are:
- Acids
- Bases, and
- Salts
In this chapter, we will study these acids, bases and salts in detail. We can check whether a substance is an acid or a base with the help of acid-base indicators. Let’s first discuss what are indicators.
What are acid-base indicators?
Acid-base indicators or just indicator a substance (dye) that changes colour or odour when we put them into an acid or a base. An indicator gives different colours in acids and bases. There are three most common indicators to identify an acid or a base. These are:
- Litmus
- Methyl orange
- Phenolphthalein
Litmus Indicator
It is the most commonly used natural acid-base indicator. It is a dye which is extracted from a type of plant called lichen. When it is neural (not acidic nor basic), then its colour is purple. It turns red in acidic solutions and blue in basic solutions. It is made into red litmus paper and blue litmus paper.
- Red Litmus Paper

- Blue Litmus Paper

Note: 1. An acid turns blue litmus to red
2. A base turns red litmus to blue
Methyl Orange Indicator
It is a synthetic acid-base indicator. The neutral colour of methyl orange is orange. It gives a red colour in acidic solution and a yellow colour in basic solution.
Phenolphthalein Indicator
It is a synthetic acid-base indicator. The neutral colour of phenolphthalein is colourless. It is also colourless in acidic solution but gives pink colour in basic solution.
Turmeric
Turmeric is also a natural indicator. It gives a red colour in the basic solution.
Red Cabbage Indicator
It is extracted from red cabbage leaves. It is red and remains red in the acidic solution but turns green in the basic solution.
Olfactory Indicator
The indicators whose odour or smell changes in acidic or basic solutions are called olfactory indicators.
Examples:
- Onion Extract
- Vanilla Extract
You know that onion has a characteristic smell. When we put the base solution on a cloth strip treated with onion extract, the onion smell cannot be detected. While the acidic solution does not destroy the onion smell. So, onion extract can be used as a test for acids and bases.
Similarly, vanilla extract has an unpleasant smell. When we put a base solution on a cloth strip treated with vanilla extract, the vanilla smell could not be detected. While the acidic solution does not destroy the vanilla smell. So, vanilla extract can be used as a test for acids and bases.
Acids
Some fruits like lemon have a sour taste due to the presence of acids. So, acids are the substances which have a sour taste but never try to taste mineral acids like hydrochloric acid, sulphuric acid and nitric acid because these are corrosive and burn the skin or body parts. We can also identify whether the given substance is acidic or not with the help of some indicators. For example, Acids change the colour of blue litmus to red.
Types of acids
Generally, based on origin acids are of two types:
- Organic Acids
- Mineral Acids
Organic Acids: The acids present in plant materials and animals are called organic acids. Some examples of organic acids are Acetic acid, Lactic acid, Citric acid, Tartaric acid, Oxalic acid, and Formic acid. They are generally weak acids.
Mineral Acids:
The acids which are prepared from the minerals of the earth are known as mineral acids. These are man-made acids. The main mineral acids are hydrochloric acid, nitric acid and sulphuric acid. They are strong acids except for carbonic acid.
These mineral acids are very dangerous and can burn our body parts and clothes if not handled carefully.
Concentrated and Dilute Acids
The acids which have the minimum possible amount of water in them are called concentrated acids. On the other hand, the acids which have much more amount of water in them are called dilute acids. The dilute acids can be prepared by adding the desired amount of concentrated acid into the desired volume of water.
What is the right way of diluting acids?
A dilute acid is prepared by mixing the desired amount of concentrated with the desired volume of water. The dilution of any concentrated acid should always be done by adding concentrated acid to the water gradually with continuous stirring.
The mixing of concentrated acid with water is a highly exothermic process (Heat producing). So, when we mix acid with water, a large amount of heat is generated.
Warning! We should never add water to concentrated acid.
Reason: 1. When we add water to concentrated acid to dilute it, then a huge amount of heat is produced at once. This heat changes some amount of water into steam explosively which can splash acid on our body and clothes and cause acid burns. Even this excessive heat may break the glass container.
But when we add concentrated acid to the large amount of water, then the heat is formed gradually, and easily absorbed by the large amount of water.
What do all acids have in common?
All acids have some common properties. Based on these properties we can say that the substance is acids. These properties are
- The taste of acids is sour
- Acids turn blue litmus paper to red
- Acidic solutions conduct electricity
- Acids dissolve in water to produce hydrogen ions (H+)
Examples:
HCl + H2O =========> H+ (aq) + Cl– (aq)
H2SO4 + H2O =========> H+ (aq) + HSO4–(aq)
HNO3 + H2O =========> H+ (aq) + NO3–(aq)
- Acid reacts with metal to produce salt and hydrogen gas.
Examples:
- Reaction of Zinc metal with Sulphuric acid: Zinc metal reacts with sulphuric acid to form zinc sulphate and hydrogen gas.
Zn (s) + H2SO4 =========> ZnSO4 + H2
- Reaction of Magnesium metal with Sulphuric acid: Magnesium metal reacts with sulphuric acid to form magnesium sulphate and hydrogen gas.
Mg (s) + H2SO4 =========> MgSO4 + H2
- Reaction of Sodium metal with hydrochloric acid: Sodium metal reacts with hydrochloric acid to form sodium chloride and hydrogen gas.
Na (s) + HCl =========> NaCl + H2
6. Reaction with Metal carbonates: Acids can react with metal carbonates to form salt, water and carbon dioxide gas.
Acid + Metal Carbonates =========> Salt + H2O + CO2
Examples:
- Sodium carbonate reacts with hydrochloric acid to form sodium chloride, water and carbon dioxide.
Na2CO3 + HCl =========> NaCl + H2O + CO2
- Potassium carbonate reacts with sulphuric acid to form potassium sulphate, water and carbon dioxide.
K2CO3 + H2SO4 =========> K2SO4 + H2O + CO2
- Calcium carbonate reacts with sulphuric acid to form calcium sulphate, water and CO2.
CaCO3 + H2SO4 =========> CaSO4 + H2O + CO2
7. Reaction with Metal hydrogen carbonates: Acids can react with metal hydrogen carbonates to form salt, water and carbon dioxide gas.
Acid + Metal Hydrogen Carbonates =========> Salt + H2O + CO2
Examples:
- Sodium hydrogen carbonate reacts with hydrochloric acid to form sodium chloride, water and carbon dioxide.
NaHCO3 + HCl =========> NaCl + H2O + CO2
- Potassium carbonate reacts with sulphuric acid to form potassium sulphate, water and carbon dioxide.
KHCO3 + HCl =========> KCl + H2O + CO2
8. Neutralization Reaction
When an acid reacts with a base, then salt and water are formed this reaction is called a neutralization reaction.
Acid + Base =========> Salt + Water
Example:
The reaction between hydrochloric acid and sodium hydroxide.
NaOH (aq) + HCl (aq) =========> NaCl (aq) + H2O
In this reaction, sodium hydroxide reacts with hydrochloric acid to form sodium chloride and water.
9. Reaction of acids with metal oxides
Acids react with metal oxides to form salt and water.
Acid + Base =========> Salt + Water
Example:
Hydrochloric acid reacts with copper oxide to form copper chloride and water.
2HCl + CuO =========> CuCl2 + H2O
10. Acids are corrosive
The mineral acids are corrosive because they cause severe burns on the skin. These acids attack and eat up materials like wood, clothes, metals, marble. We should be careful while using these acids in the laboratory.
Important question:
Why acids are never stored in metal containers?
Ans.: Acids are never stored in metal containers because they gradually corrode and eat up the metal container.
Note: Acids are stored in containers made of glass and ceramics because acids are not attacked by acids. A hazard warning sign is usually printed on their containers.
The hazard warning sign on the acid containers is shown as :

Question: Why do acids not show acidic behaviour in the absence of water? Explain with an example.
Answer: Acids do not show acidic behaviour in the absence of water because they will not form hydrogen ions in the absence of water.
Example: Dry HCl gas does not show acidic behaviour.
When we added conc. sulphuric acid in sodium chloride, then dry HCl gas is formed. The dry HCl gas does not change the colour of dry blue litmus paper because it has no hydrogen ions. But, when dry HCl gas comes in contact with wet blue litmus paper, then blue litmus paper turns to red. It is because when dry HCl gas comes in contact with wet litmus paper, it produces hydrogen ions.

Strong and Weak acids based on ionization
Based on ionization acids are of two types:
- Strong acids
- Weak acids
Strong Acids:
The acids which are completely ionized in water and produce a large amount of hydrogen ions are called strong acids.
Examples:
- Hydrochloric acid (HCl)
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
These acids are completely ionized in water and produce large amounts of hydrogen ions.
- HCl + Water =========> H+(aq) + Cl–(aq)
- H2SO4 + Water =========> H+(aq) + HSO4–(aq)
- HNO3 + Water =========> H+(aq) + NO3–(aq)
Weak Acids:
The acids which are not completely ionized (Partial ionization) in water and thus produce a small amount of hydrogen ions are called weak acids.
Examples:
- Carbonic acid (H2CO3)
- Acetic acid (CH3COOH)
- Sulphurous acid (H2SO3)
These acids are not completely ionized in water and produce small amounts of hydrogen ions.
CH3COOH + Water =========> H+(aq) + Cl–(aq)
Bases
The aqueous solutions of some bases like lime, washing soda and caustic soda are soapy in touch, and bitter in taste (in Hindi- Kadwa Swad). Therefore, the bases are chemical substances which have a bitter taste and are soapy in touch. They can also be identified with the help of a litmus paper test.
Litmus Paper Test
The bases change the colour of red litmus paper to blue.

All metal oxides, hydroxides, carbonates and hydrogen carbonates are bases.
Examples:
- Calcium oxide (CaO)
- Magnesium Oxide (MgO)
- Potassium oxide (K2O)
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide [Ca(OH)2]
- Ammonium hydroxide (NH4OH)
- Sodium carbonate (Na2CO3)
- Sodium hydrogen carbonate (NaHCO3)
- Potassium hydrogen carbonate (KHCO3)
Alkalis
The bases which are soluble in water are called alkalis. The water-soluble alkalis are:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide [Ca(OH)2]
- Magnesium hydroxide [Mg(OH)2]
- Ammonium hydroxide (NH4OH)
All alkalis produce hydroxide ions (OH–) when dissolved in water.
Types of bases (alkalis) based on ionization
Based on ionization bases are of two types:
- Strong bases
- Weak bases
Strong bases:
The bases which are completely ionized in water and produce a large amount of hydroxide ions are called strong bases.
Examples:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
These bases are completely ionized in water and produce large amounts of hydroxide ions.
- NaOH + Water =========> OH–(aq) + Na+ (aq)
- KOH + Water =========> OH–(aq) + K+ (aq)
- Mg(OH)2 + Water =========> OH–(aq) + 2Mg+ (aq)
Weak Bases:
The bases which are not completely ionized (Partial ionization) in water and thus produce a small amount of hydroxide ions are called weak bases.
Examples:
- Magnesium hydroxide [Mg(OH)2]
- Ammonium hydroxide (NH4OH)
- Calcium hydroxide [Ca(OH)2]
These bases are not completely ionized in water and produce a small amount of hydroxide ions.
- Mg(OH)2 + Water =========> OH–(aq) + 2Mg+ (aq)
- Ca(OH)2 + Water =========> OH–(aq) + 2Ca+ (aq)
- NH4OH + Water =========> OH–(aq) + NH4+ (aq)
Properties of bases:
- Bases have a bitter taste
- These are soapy in touch
- Bases turn red litmus to blue
- Bases (alkalis) produce hydroxide ions when dissolved in water.
- The aqueous solution of these bases conducts electricity.
- They produce hydrogen gas when react with some metals.
Example:
Sodium hydroxide reacts with zinc metal to form sodium zincate and hydrogen gas.
NaOH + Zn =========> Na2ZnO2 + H2
Sodium Zincate
- Bases react with acids to form salt and water.
Example:
The reaction between hydrochloric acid and sodium hydroxide.
NaOH (aq) + HCl (aq) =========> NaCl (aq) + H2O
In this reaction sodium hydroxide reacts with hydrochloric acid to form sodium chloride and water.
- Bases react with non-metal oxides to form salt and water.
Example:
Calcium hydroxide reacts with carbon dioxide to form calcium carbonate and water.
Ca(OH)2 + CO2 =========> CaCO3 + H2O
pH Scale
The strength of acid and base solutions
Pure water is neutral (neither basic nor acid) because it contains an equal number of hydrogen ions (H+) and hydroxide ions (OH–).
However, when we dissolve an acid into water the concentration of hydrogen ions increases, while when we dissolve a base into water the concentration of hydroxide ions increases. So, acidic solutions have more concentration of hydroxide ions in comparison to hydroxide ions and basic solutions have more concentration of hydroxide ions in comparison to hydrogen ions.
The Sorenson, in 1909 developed a scale by making the use of hydrogen ion concentration. This scale is known as the pH scale. In the term pH, the letter p stands for the German word “potenz” means power and the letter H stands for hydrogen ion concentration.
With the help of the pH scale, we can determine the strength of an acid or a base. The value of pH starts from number 0 to 14 and it has no unit.
There is a relationship between the pH scale and hydrogen ion concentration. The pH of a solution is inversely proportional to the hydrogen ion concentration.
The neutral substances have a pH value of exactly 7. The acids have a pH value of less than 7, while bases have a pH value of more than 7.
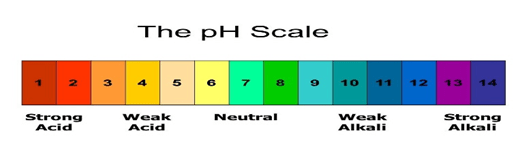
The pH scale is shown below:
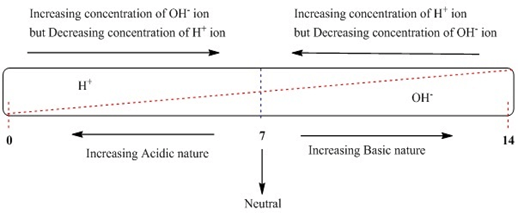
It can also be represented as:
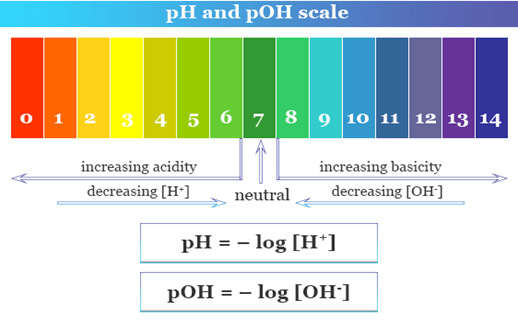
Importance of pH in everyday life
In our day-to-day life, pH plays an important role in many activities. Some of the uses of pH are discussed here:
- pH role in our digestive system:
Hydrochloric acid produced by our stomach helps in the digestion of food without harming the stomach. Sometimes, it is produced in the access amount due to various reasons and causes indigestion which produces pain and irritation. This is cured by using antacids like magnesium hydroxide (Milk of magnesia) and sodium hydrogen carbonate (baking soda).
- Tooth Decay due to the change in pH value. The tooth decay starts when the pH of acid in the mouth falls below 5.5.
- Animals and plants are sensitive to pH changes
Both plants and animals grow well in the proper conditions of pH. If the pH of the soil is too acidic or too basic then the plants grow badly. The pH of the soil is affected by the use of chemical fertilizers. The pH of the acidic soil can reach as low as 4 and of the basic soil can reach up to 8.3.
The pH also affects the growth, development and survival of animals including human beings.
- Self-defense by plants and animals:
Many plants and animals protect themselves from their enemies by injecting painful and irritating chemicals including acids and bases into their skin. Example: Wasp inject an alkaline liquid while a honey bee injects acidic liquid into the skin of a person causing burning pain. Similarly, nettle plant hair present on the leaves injects methanoic acid into the skin and causes burning pain.
Salts
Salts are compounds formed by the reaction between an acid and a base. For example: Sodium chloride is formed by the reaction of hydrochloric acid with sodium hydroxide.
HCl + NaOH =========> NaCl + H2O
Naming of salts:
- The salts of hydrochloric acid are called chlorides.
- The salts of nitric acid are called nitrides.
- The salts of sulphuric acid are called sulphides.
- The salts of acetic acid are called acetates.
- The salts of carbonic acid are called carbonates.
Examples of salts
- Sodium chloride (NaCl)
- Potassium Chloride (KCl)
- Magnesium Chloride (MgCl2)
- Zinc Chloride (ZnCl2)
- Calcium Chloride (CaCl2)
- Sodium sulphate (Na2SO4)
- Potassium sulphate (K2SO4)
- Magnesium sulphate (MgSO4)
- Calcium sulphate (CaSO4)
- Aluminium sulphate (Al2(SO4)3
- Sodium nitrate (NaNO3)
- Zinc carbonate (ZnCO3)
- Sodium acetate (CH3COONa)
The pH of Salt Solutions
- The salts of strong acids and strong bases give neutral solutions (pH=7).
Examples: Sodium chloride and Potassium sulphate.
- The salts of weak acids and strong bases give basic solutions (pH more than 7).
Example: Sodium carbonate and Potassium carbonate
- The salts of strong acids and weak bases give neutral solutions (pH less than 7).
Example: Ammonium chloride
Common Salt (Sodium Chloride)
The chemical name of common salt is sodium chloride. It is a neutral salt because it is formed by strong acids and strong bases give neutral solutions.
HCl + NaOH =========> NaCl + H2O
Natural Sources of common salts
The natural resources of common salts are sea water and rock salt.
Uses of Common salt
- In the manufacturing of many useful chemicals such as sodium hydroxide, sodium carbonate, hydrochloric acid, chlorine, and sodium metal.
- It is used in cooking food.
- It is used in the manufacturing of soap.
- It is used to melt ice which collects on the road in cold countries.
Manufacturing of Sodium hydroxide
It is prepared by the electrolysis of concentrated aqueous solution of sodium chloride. It is formed near the cathode with hydrogen while chlorine gas is produced at the anode.
NaCl + H2O =========> NaOH + H2 + Cl2
This process is also known as the Chlor-Alkali Process because of the products formed.
Uses of Sodium Hydroxide: In making soap, detergents, paper, artificial fibres such as rayon etc.
Manufacturing of Washing Soda
It is also known as sodium carbonate decahydrate (Na2CO3.10H2O). It is produced from sodium chloride in the following three steps:
Step 1: The reaction of concentrated and cold brine solution with ammonia and carbon dioxide to obtain sodium hydrogen carbonate.
NaCl + H2O + NH3 + CO2 =========> NaHCO3 + NH4Cl
Step 2: The sodium hydrogen carbonate on heating decomposes to form sodium carbonate.
NaHCO3 =========> Na2CO3 + CO2 + H2O
Step 3: Sodium carbonate dissolves in water and recrystallized to form washing soda.
Na2CO3 + H2O =========> Na2CO3.10H2O
Uses of Washing Soda:
- In making glass, paper, and soap.
- It is used as a cleansing agent.
- It is used in making sodium compounds such as borax.
Manufacturing of Baking Soda
It is also known as sodium hydrogen carbonate decahydrate (NaHCO3). It is produced from sodium chloride. The reaction of concentrated and cold brine solution with ammonia and carbon dioxide to obtain sodium hydrogen carbonate.
NaCl + H2O + NH3 + CO2 =========> NaHCO3 + NH4Cl
Uses of Baking Soda:
- It is used as an antacid to remove the acidity of the stomach.
- It is used in making cakes, breads etc.
- It is used in fire extinguishers. It is produced carbon dioxide by the reaction of dilute sulphuric acid.
2NaHCO3 + dil H2SO4 =========> Na2SO4 + CO2 + H2O
Other Useful Salts
- Bleaching Powder (CaOCl2)
It is also known as calcium oxychloride. It is prepared by passing chlorine gas over dry slaked lime.
Ca(OH)2+ Cl2 =========> CaOCl2 + H2O
The bleaching powder reacts with a dilute acid to form chlorine gas (the bleaching agent present in the bleaching powder).
Uses of Bleaching Powder:
- It is used for the bleaching of cotton and linen in the textile industry.
- It is used for the bleaching of wood pulp in the paper industry.
- It is used for disinfecting drinking water.
- It is used in making chloroform.
- It is used for making wool unshrinkable.
- It is used as an oxidizing agent.
- Plaster of Paris (CaSO4. H2O)
Chemically, it is calcium sulphate hemihydrate. It is commonly known as P.O.P. It is prepared by heating gypsum (CaSO4.2H2O) at 100°C (373K).
CaSO4.2H2O =========> CaSO4. H2O + 1 H2O
It can also be written as 2CaSO4.H2O.
Properties of Plaster of Paris:
- It is a white powder.
- It sets into a hard mass wetting with water due to the formation of gypsum.
CaSO4. H2O + H2O =========> CaSO4.2H2O
Uses of Plaster of Paris:
- It is used in making toys, cheap ornaments, chalk, casts for statues, and cosmetics.
- It is used as a fire-proofing material.
- It is used in hospitals for setting fractured bones in the right positions to ensure correct healing.
- It is also used in making designs on the ceilings of houses.
- Hydrated Salts
The salts which contain water of crystallization are called hydrated salts. Each hydrated salt has a fixed number of molecules of water of crystallization in its unit formula. The water of crystallization is not free so, it does not wet the salt.
Examples:
- Copper sulphate pentahydrate (CuSO4.5H2O).
- Calcium sulphate dihydrate (CaSO4.2H2O)
- Iron sulphate heptahydrate (FeSO4.7H2O)
- Sodium carbonate decahydrate (Na2CO3.10H2O)
Properties:
- Colour: These are generally coloured due to the presence of crystallized water. For example, Copper sulphate pentahydrate (Blue), Iron sulphate heptahydrate (green), and Calcium sulphate dehydrate (white).
- They lose their water of crystallization on strong heating and lose their regular shape and color. The salts which have lost their water of crystallization are called anhydrous salts.
Author: Dr. Vinesh Kumar (Ph.D. Chemistry)
Support Us: Please Donate to Support Us
Scan QR to Donate

Suggesting Post